PEMF Therapy: What Consumers Need to Know
History, studies and making your own informed choices. All covered in this article.
By PEMF Mat Reviews content team
Updated: Nov 18, 2020
History: Humans and Magnetic Fields
In Ancient Greece, Egypt, India, and China, prior to scientific reasoning enabling the understanding of the benefits of magnetic fields, lodestones (magnetized stones) were widely incorporated into medical therapies. There is some evidence that popular historic figures such as Cleopatra and Hippocrates used magnets and magnetised stones in order to combat pain and preserve youth. The use of magnets and magnetised stones continued, relatively unbacked by science until the 19th century, where Michael Faraday proposed that a changing magnetic field produced an electric field (also known as Faraday’s law, which we write about in our news post here).
Recent History
Popular 20th century inventor and scientist Nikola Tesla was a gifted innovator that had a strong grasp of magnetic fields and electricity. His knowledge led to many inventions, including contributions towards the development of the AC (Alternating Current) electrical system and the museum-popularized Tesla coil. As we discussed on an old blog post on Tesla, he discovered through his research that passing electrical currents through the human body was harmless. His contribution to the development of the understanding of magnetic fields and electricity built the basis of much research and development of PEMF devices today.
Further into the 20th century, magnetic therapies and PEMF devices were being developed at a greater pace, specifically in European countries. The 1980s were a crucial time period for the development of PEMF devices, as the first FDA-approved device was manufactured, for the purpose of stimulating the bone to aid in the recovery of fractures. Around this time, books and research papers started to emerge classifying the body as an electromagnetic object that therefore was affected by magnetic fields (building on Faraday’s Law – see book: Body Electric for more).
As press built around the research going on regarding the effects of magnetic fields on humans, inquires regarding the use of magnetic devices, specifically PEMF devices, in space began to emerge. This was due to the misconception that humans in space were outside Earth’s magnetic field. The potential application of PEMF devices that came from this misfounded discussion was the use of PEMF devices to mimic Earth’s magnetic field in human deep space travel in the future .
PEMF Today
As more devices and products using PEMF therapy are being produced, there has been an increasing volume of research surrounding PEMF technology, as people seek to validate the claims of manufacturers that are selling products often for thousands of dollars. The research is also undertaken to built a body of knowledge on the potential benefits and side effects of this new technology that for the most part, has not been scientifically proven. A large portion of positive results in studies on pubmed support the use of high intensity PEMF devices to treat specific ailments. Most PEMF products on the market today are low intensity. Companies claim that low intensity is superior to high intensity, and for certain health issues they are correct, however it is easier in general to market (no FDA approval needed), sell, and produce lower intensity products and sell at a high markup, which is why many companies choose to produce at low intensity.
What exactly is PEMF Therapy?
PEMF Therapy stands for Pulsed Electromagnetic Field Therapy, and at its current state, is the cumulative result of historic use and research on the relationship between magnetic fields and electricity (see above). As was discovered by Faraday, changing a magnetic field generates an electric field, and the same principle works the other way around. PEMF Therapy is administered today through coils, either directly, or embedded in a mat, applicator etc. The material used in these coils is often copper, as copper allows electricity (that is powering the PEMF device) to flow effectively through the coils. This flow of electricity through the coils generates magnetic fields, which is why the “EMF” in “PEMF” is called Electromagnetic Field: the magnetic field is generated by the movement of electricity (Electro > Magnetic).
How does PEMF Therapy interact with the human body?
The human body is composed of around 37 trillion cells, and more than 200 different cell types. Each cell in the body requires energy to operate, and this energy requires ATP (Adenine Triphosphate). The reason ATP is required by the cell for energy, is that ATP transports chemical energy within the cells. When Electromagnetic fields from PEMF devices pass through the body, they generate additional ion motion, therefore generating a greater electrical field within the body, and energizing cells. Different types of PEMF therapy may be beneficial to different types of cells (from the 200 or so varieties), which is why we stress that there is no best type of PEMF therapy. By affecting the charge of the cell, specifically the cell membrane, the magnetic fields passing through the body increase the level of transportation and waste excretion in cells.
Is PEMF Safe to use?
The thought of electromagnetic fields passing through the body may be scary for some people, but the truth is that we are exposed to these types of fields everyday, in the form of cell towers, microwaves, digital devices etc. Any connection that you cannot physically see occurs through these invisible fields, with different devices communicating at different frequencies. Traditional EMFs that emit from cellular devices, digital display etc. have occasionally been reported to negatively affect human beings. However, the current consensus around EMFs is that they are for the most part not harmful or dangerous at low levels, evidenced by this World Health organization report.
Further to this, PEMF is different from traditional EMFs in that the devices which implement PEMF therapy operate at ranges from extremely low frequencies (ELF) to very low frequencies (VLF). Different types of EMFs operate at different frequencies, but all of the devices that have had alleged negative impact on human health have all operated at frequencies much higher than any PEMF device.
Clinical studies and research shows that PEMF therapy is a safe form of non-invasive therapy, and the FDA has ruled that low frequency, low intensity PEMF devices do not have to be FDA approved to be marketed in USA. This means that according to the FDA, PEMF devices are safe enough that they do not have to undergo the rigorous testing that many drugs and medical devices have to go through prior to being available to consumers.
OK it's safe, but what is so good about PEMF Therapy?
There are countless studies that have been conducted which show the benefits of PEMF therapy using various devices and variables. The two main benefits that have emerged from these studies are improvements in general health and temporary pain relief. There have been several extraordinary results using PEMF therapy, such as curing certain diseases, however these outcomes have not been replicated to a significant enough level that generalized health claims can be made.
What makes PEMF therapy such an attractive option is not only the potential health benefits that come out of it, but the convenience and medium by which the therapy is provided. The main type of application of PEMF therapy comes in the form of mats, with coil based devices also extremely popular. By sitting or lying down in a resting position, PEMF therapy can be administered in a non-painful and relaxed manner, often resulting in great health outcomes. The following four outcomes have been most stated as common benefits of PEMF therapy:
Higher rate of injury healing
Improved Immune function
Sleep improvement
Circulation improvement
Scientific Research on PEMF Therapy
Two good resources to use to view scientific studies & research on PEMF therapy are pubmed.gov and Google Scholar. Simple queries of “PEMF therapy” on either resource center will show you some of the trials conducted with PEMF therapy. Below is a list we have put together a list on some benefits noted from citations on both pubmed and Google, along with links to those citations. This list contains the top 4 studies that we found, on the basis that they are human based, and were conducted with a sample size that is generally considered statistically significant. Read more below to see how to evaluate scientific studies on your own.
- Management of Osteoarthritis-related pain, stiffness and physical function in the elderly
- Pain reduction and increased circulation post plastic surgery
- Regulation of blood pressure and circulation of nitric oxide levels in individuals with metabolic syndrome
- Augmentation to pharmacotherapy in treatment of treatment-resistant depression
The Stats & Methodology behind PEMF Studies
Evaluating your own studies is one of the best things to do prior to purchasing a PEMF product. PEMF studies validate some of the claims that manufacturers of PEMF products, or health clinics make. It is key to note that PEMF studies, while extensively researched, do not have hundreds of studies that measure the exact same therapeutic result, meaning reliability is at best, uncertain. You will notice, especially in Pubmed, that the information presented is in abstract form, meaning the full study is not shown. Usually the abstract is good enough for consumers to evaluate the study, with the full study really only beneficial to those looking to dive deep into the details of individual trials. Below is a sample abstract that will serve as an example for us to show you how to evaluate studies.
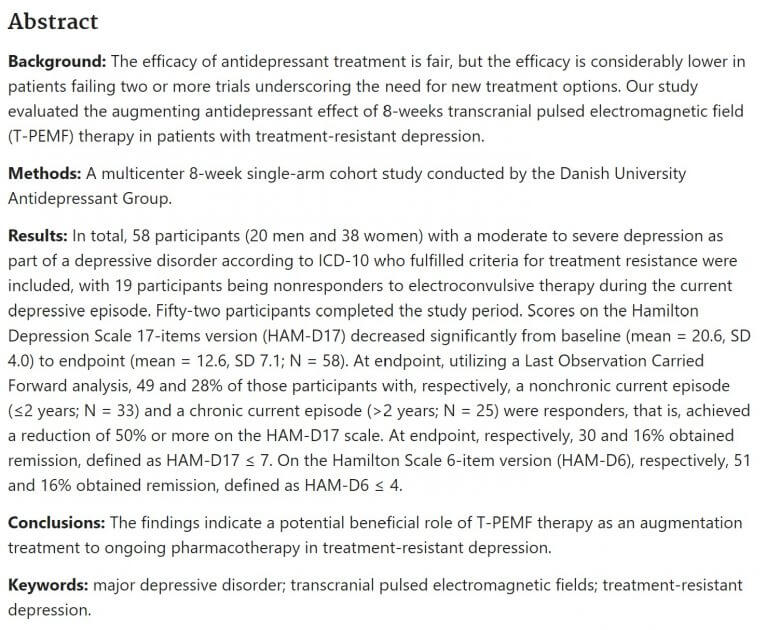
Background: The background section explains why the study was undertaken in the first place. With PEMF therapy, it generally refers to a generalized treatment that is currently being used to treat a condition which works, but alternative healing methods are being explored.
Methods: The methods section explains how the study was conducted. This includes the length of study, implementation approach to the study, and which person/group administered the study. Length of study varies dependent on the results that are sought after from the initiation of the study. The implementation approach is at the discretion of the administration. The following are some common implementation approaches:
Single arm study: Participants are selected based off volunteers for the study, and all participants receive the same treatment
Double arm study: Participants are selected based off volunteers for the study, with participants receiving two separate treatments (group 1, group 2)
Single blind study: Participants are unaware if they are receiving treatment or placebo
Double blind study: Both participants and experimenters are unaware if they are receiving/administering treatment or placebo
Generally speaking, a double blind study is the best type to conduct because it reduces experimenter and participant bias, meaning more objective results, however, the other study types are effective based on the other variables within the method of the study.
Results: The results section is often the most confusing to interpret, given the stats that are described within the briefing. We will break down some of the most common statistical terms and abbreviations that are used, so you know what to look for in the results briefing.
n = Sample size AKA number of participants. Generally speaking, you want to have a sample size over 30 to extrapolate data from the study (based on the central limit theorem in statistics – click here to learn more).
Mean = Average result
SD = Standard deviation = variation of a set of values. Greater SD means more variation in the results of the study.
p = p-value = probability of obtaining results at least as extreme as those observed if the test is conducted as a statistical hypothesis, under the assumption that the null hypothesis (no relationship between 2 variables) is correct. Any value stated at p < 0.05 is statistically significant, meaning that the null hypothesis can be rejected in most cases (ex. the null hypothesis that there is no relationship between PEMF therapy and pain reduction can be rejected if the results of the statistical hypothesis-based study contains a p-value < 0.05 – in most cases, unless the administrator sets the p-value threshold below 0.05, which is extremely rare).
If you don’t want to get into the details of the results, looking at the sample size and mean will give you a high level understanding of the results of the study.
Conclusions: This is where to go if you want a quick synopsis and to get the final judgement based on the study.
I have done my research, how can I get PEMF therapy?
General consumers: You can get PEMF therapy at a variety of health clinics, such as Chiropractor offices. Sessions with PEMF products at these clinics can run between $30 – $100 per hour. Alternatively you can purchase your own PEMF therapy products online. Here at pemfmatreviews, we specifically review PEMF mats available to purchase on our site under the “mat reviews” section. We make reviews to better inform consumers on which product is best suited for for their needs. PEMF products are not registered medical devices, so anyone can purchase them.
Professionals: If you are a registered medical professional or health field worker, you can often purchase PEMF products at a discount. Many consumer based PEMF product companies offer a “professional” product option, aimed at people looking to use products in their practice. The professional product lines are also great options for consumers.
Veterinarians / Horse owners: Companies such as BEMER offer equine solutions, with custom products designed specifically for horse performance and healing. Most PEMF products are safe to use on humans and animals, making them a great choice for pets and veterinarians to use in their practice.
Evaluating your PEMF product options
Low vs High Intensity PEMF devices
There are arguments for both High and Low intensity devices and their effectiveness, but as we have stated before, there is really no right answer here, as it depends on the needs of the user. One key consideration to note however, is that magnetic field intensities decrease (drop off) extremely rapidly from the surface of a PEMF coil. This means that the deeper the magnetic field goes into the body and its tissues, the lower the intensity. With low intensity devices, there is a requirement for longer sessions, and more continuous treatment as opposed to high intensity devices as a result.
High intensity devices have a greater chance of reaching affected tissue, and therefore could be a better option for specific health issues. However, if the intensity of a PEMF device reaches extremely high levels, there could be potential for side effects (largely unknown currently). The following summarizes pros and cons of both types of intensities. Prior to examining these, remember that with Pulsed EMFs, the combination of frequency (pulse) and intensity (EMF field) determine the “safety” of the therapy.
Low Intensity: Low intensity PEMF devices are readily available and widely marketed to consumers. Their price ranges from $500 to around $10,000. Low intensity devices are usually between 1 – 10 Gauss, with a wide range of frequencies at those intensity levels. The main benefits of low intensity devices is the ability to operate at various frequencies in and around the natural magnetic field of the Earth. These types of devices follow the intensities and frequencies that have been publicly researched and tested, specifically on pubmed. The one downside to low PEMF intensity is the lack of potential to assist with more serious or specific health ailments.
High Intensity: High intensity PEMF devices are less available and marketed to consumers, due to the stricter requirements by governing bodies such as the FDA. Their prices can go past $30,000, and often are only available to licensed professionals, but some are sold directly to consumers. The benefits of high intensity devices are the ability to potentially assist with specific health ailments due to the ability to reach deeper into the body. The main concern with high intensity devices is their safety.
High intensity PEMF devices have received FDA approval, but some have been banned, and even caused patient death. High intensity devices are extensively researched and tested to receive FDA approval, but outside this, are not generally as researched or peer reviewed on publicly available online resources. Many companies that produce high intensity PEMF devices state their safety by referencing MRI machines, which often have magnetic intensity of greater than 15,000 Gauss for imaging capabilities. However, the magnetic fields of MRI machines are static, and not pulsed, which is where high intensity devices can run into issues, if they combine high intensity with high frequency.
There is immense potential for high intensity PEMF devices, but currently, there is not enough publicly available research to back up some of the health claims that are being made by companies, and there are definitely health concerns if these devices are able to combine high intensity with high frequency.
501K: FDA Registration vs Approval
Too often we companies & affiliates interchanging the terms FDA registered and FDA approved, often using synonyms to approval. Most low intensity devices, which are the main products we review on this site, have been deemed safe to a level that there is no need for FDA approval to market and sell the product. This being said, FDA registered means that a product has been registered with the FDA via a 510(k) form, and has been registered following a quick review by the FDA for the registered purposes of the product. FDA approved means that a product has been extensively researched and tested by the FDA. FDA approval is more common amongst devices that have potential for side effects.

Medical Disclaimer
This website does not contain medical/health advice. The medical/health information is provided for general informational and educational purposes only and is not a substitute for professional advice. Accordingly, before taking any actions based upon such information, we encourage you to consult with the appropriate professionals. We do not provide any kind of medical/health advice. THE USE OR RELIANCE OF ANY INFORMATION CONTAINED ON THIS SITE IS SOLELY AT YOUR OWN RISK.
Affiliate Disclaimer
This website may contain links to affiliate websites, and we receive an affiliate commission for any purchases made by you on the affiliate website using such links.


